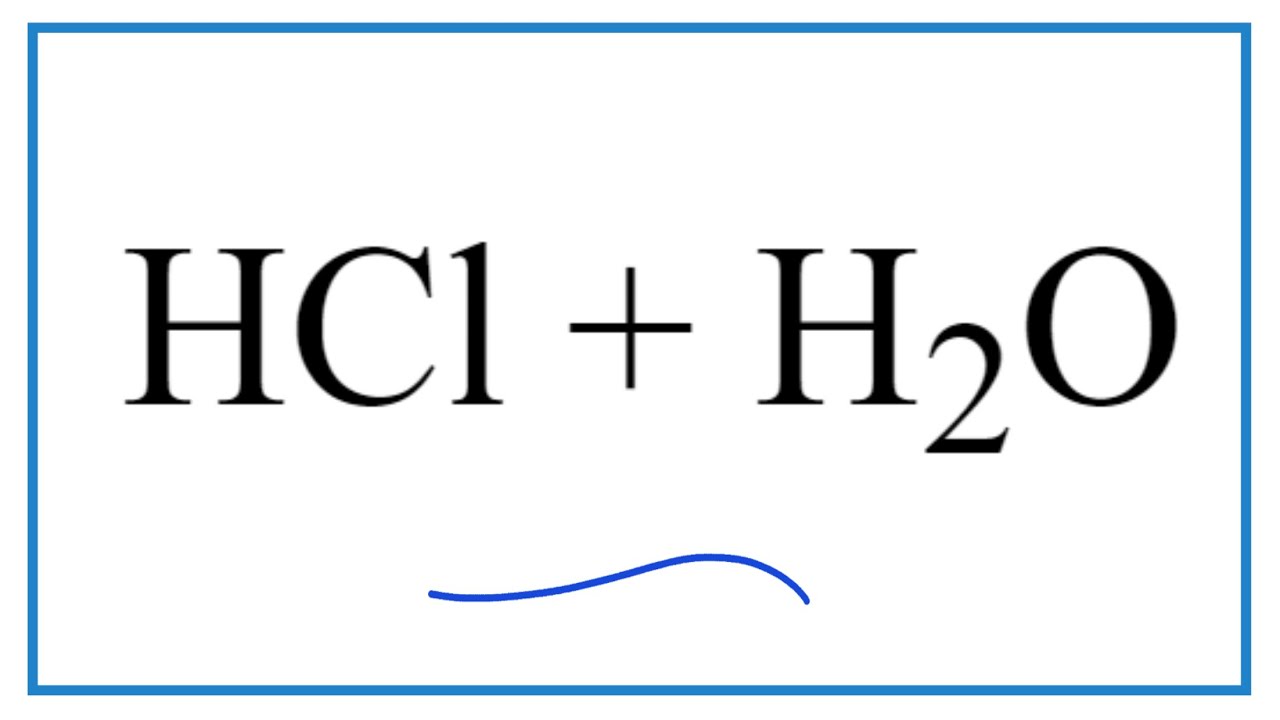Drawing Of The Reaction Of Hydrochloric Acid With Water
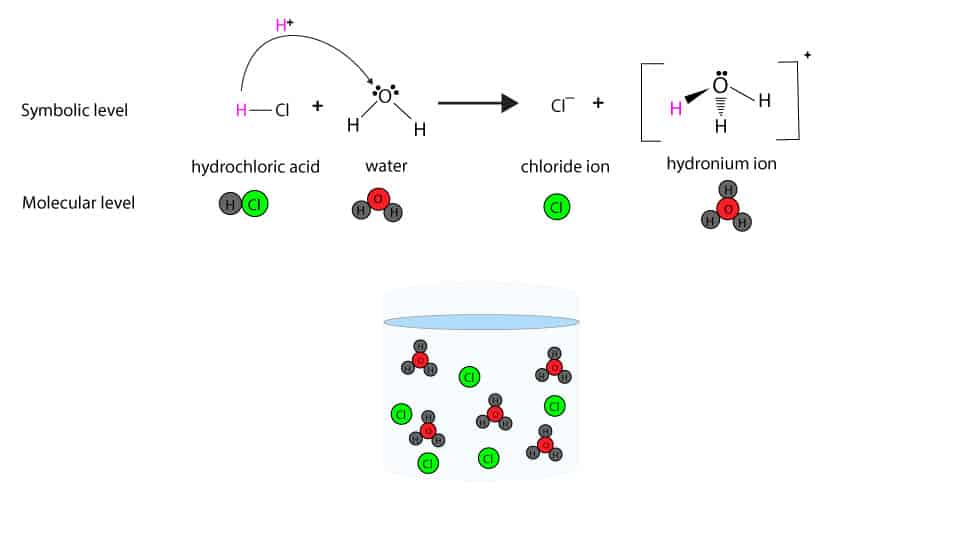
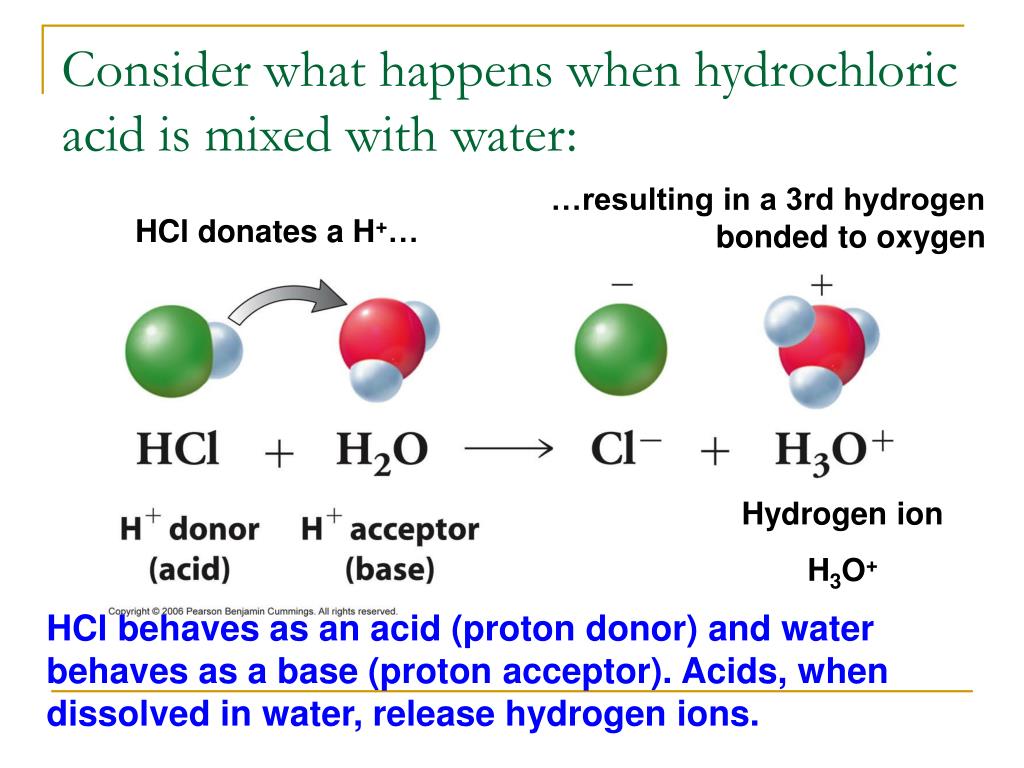
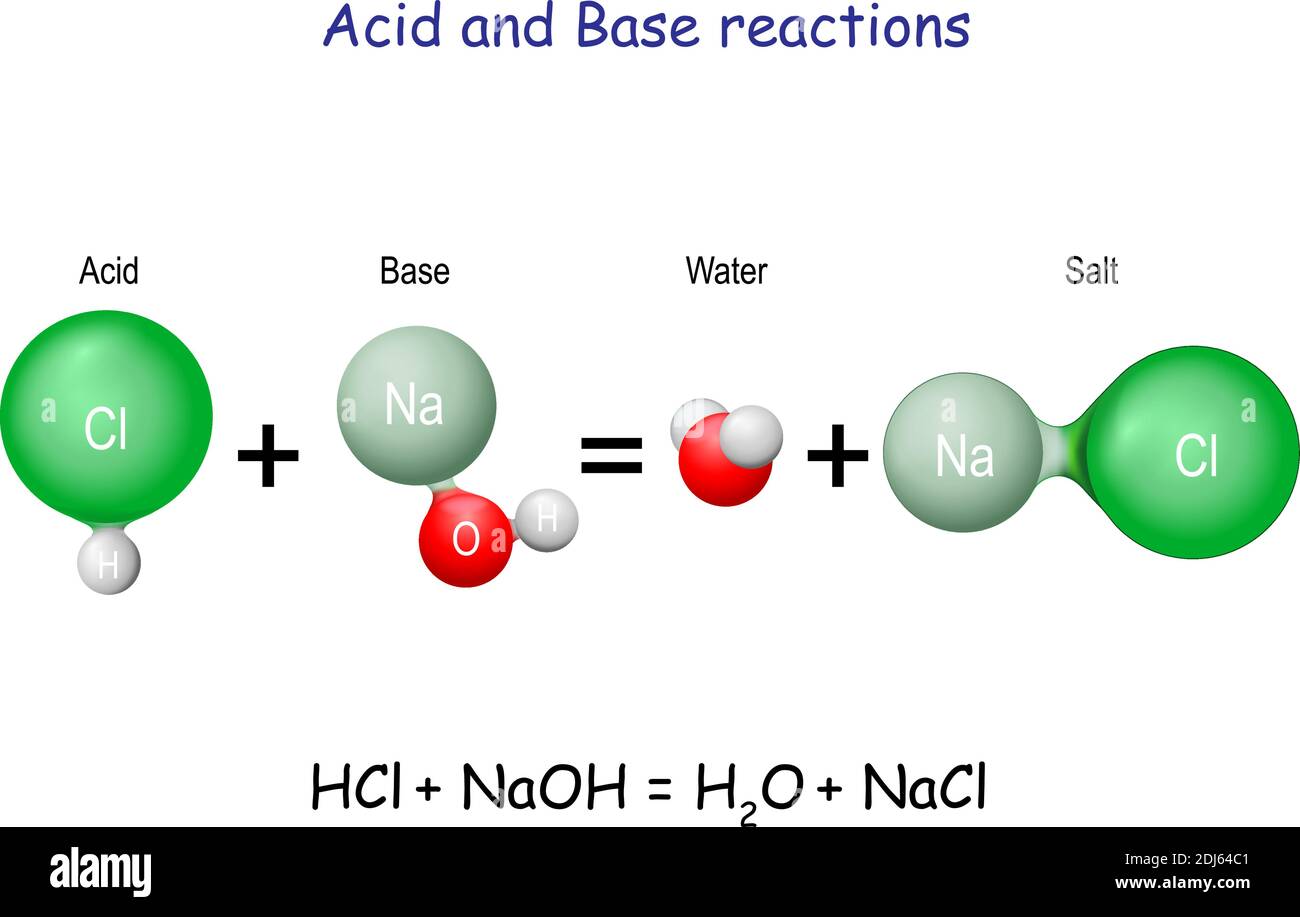
Drawing Of The Reaction Of Hydrochloric Acid With Water - The ionization of hydrochloric acid in water is given below: The acid dissociation or ionization constant, k a, is large, which means hcl dissociates or ionizes practically completely in water. (i) draw a diagram to show the arrangement used for the absorption of hcl gas in water. Hcl (aq) ⇌ h + (aq) + cl − (aq) In other words, every molecule of hydrochloric acid that is added to water will donate its proton, h+, to water molecule to form a. Hydrochloric acid is a strong acid, stronger than water, so it’ll force water to act as a base instead. Acids react with metals to produce a salt and hydrogen. Hcl(aq) hx+(aq) +clx−(aq) h c l ( a q) h x + ( a q) + c l x − ( a q) i understand that when added to water the h h leaves its electron to the cl c l atom forming the clx− c l x − and the h h attaches to water to form the hydronium ion ( hx3ox+ h x 3 o x + ). The following equation is used for calculating acid and base molarity where the concentration is given in wt %: Acid plus base yields water plus salt. Hydrochloric acid is a strong acid, stronger than water, so it’ll force water to act as a base instead. Hydrochloric acid, hcl, is a strong acid, so right from the start you should expect it to ionize completely in aqueous solution. As a general concept, if a strong acid is mixed with a weak base, the resulting solution will be slightly acidic. For example, the reaction of equimolar amounts of hbr and naoh to give water and a salt (nabr) is a neutralization reaction: For example, hydrochloric acid, hcl, as a strong acid it donates a proton to water, h2o, to form the hydronium ion, h3o plus, and the conjugate base to hcl which is the chloride anion, cl minus. If you paired water with something which is a weaker acid than water, then water will act as an acid. Use appropriate tools to draw a schematic representation of the products, showing the hydrated ions and water molecule orientation. The acid dissociation or ionization constant, k a, is large, which means hcl dissociates or ionizes practically completely in water. Hydrochloric acid is a strong acid which dissociates into h + and cl − ions in water. [ (% × d) / mw] × 10 = molarity. The above equation can then be used to calculate the molarity of the 70 wt % nitric acid: For example, the reaction of equimolar amounts of hbr and naoh to give water and a salt (nabr) is a neutralization reaction: In this video we will look at the equation for hcl + h2o and write the products. Through a process. Give two reasons for the same. Hydrochloric acid + magnesium →. The following equation is used for calculating acid and base molarity where the concentration is given in wt %: Since the h+ (often called a “proton”) and. Draw the structures of methanol (\(\mathrm{ch}_{3}\mathrm{oh}\)), acetic acid (\(\mathrm{ch}_{3}\mathrm{cooh}\)), and methane (\(\mathrm{ch}_{4}\)) and write a potential reaction with water. Hcl (aq) ⇌ h + (aq) + cl − (aq) Hydrochloric acid, hcl, is a strong acid, so right from the start you should expect it to ionize completely in aqueous solution. [ (% × d) / mw] × 10 = molarity. Give two reasons for the same. Use appropriate tools to draw a schematic representation of the products, showing. This process is a highly exothermic reaction. In reality, this reaction reaches an equilibrium. (iii) write the chemical equation for the laboratory preparation of hcl gas when the reactants are: Hydrochloric acid is prepared by dissolving gaseous hydrogen chloride in water. Hydrochloric acid, hcl, is a strong acid, so right from the start you should expect it to ionize completely. Since the h+ (often called a “proton”) and. The ionization of hydrochloric acid in water is given below: (ii) why is such an arrangement necessary? Draw the structures of methanol (\(\mathrm{ch}_{3}\mathrm{oh}\)), acetic acid (\(\mathrm{ch}_{3}\mathrm{cooh}\)), and methane (\(\mathrm{ch}_{4}\)) and write a potential reaction with water. The above equation can then be used to calculate the molarity of the 70 wt %. D = density (or specific gravity); Hydrochloric acid is a strong acid which dissociates into h + and cl − ions in water. The resulting solution is called hydrochloric acid and is a strong acid. (ii) why is such an arrangement necessary? If the base is a metal hydroxide, then the general formula for the reaction of an acid with. The resulting solution is called hydrochloric acid and is a strong acid. A class practical on reacting magnesium with hydrochloric acid and how to measure the rate of reaction. The ionization of hydrochloric acid in water is given below: Hydrochloric acid is prepared by dissolving gaseous hydrogen chloride in water. In other words, every molecule of hydrochloric acid that is. Here’s the best way to solve it. [ (% × d) / mw] × 10 = molarity. If you paired water with something which is a weaker acid than water, then water will act as an acid. Since the h+ (often called a “proton”) and. Draw the structures of methanol (\(\mathrm{ch}_{3}\mathrm{oh}\)), acetic acid (\(\mathrm{ch}_{3}\mathrm{cooh}\)), and methane (\(\mathrm{ch}_{4}\)) and write a. The above equation can then be used to calculate the molarity of the 70 wt % nitric acid: This reaction highly favors the formation of products, so the reaction arrow is drawn only to the right. If the base is a metal hydroxide, then the general formula for the reaction of an acid with a base is described as follows:. Since the h+ (often called a “proton”) and. For example, hydrochloric acid, hcl, as a strong acid it donates a proton to water, h2o, to form the hydronium ion, h3o plus, and the conjugate base to hcl which is the chloride anion, cl minus. The above equation can then be used to calculate the molarity of the 70 wt %. Hydrochloric acid is a strong acid which dissociates into h + and cl − ions in water. Acid plus base yields water plus salt. The ionization of hydrochloric acid in water is given below: Since the h+ (often called a “proton”) and. Mw = molecular weight (or formula weight). A class practical on reacting magnesium with hydrochloric acid and how to measure the rate of reaction. D = density (or specific gravity); Hcl (aq) ⇌ h + (aq) + cl − (aq) In other words, every molecule of hydrochloric acid that is added to water will donate its proton, h+, to water molecule to form a. Hcl(aq) hx+(aq) +clx−(aq) h c l ( a q) h x + ( a q) + c l x − ( a q) i understand that when added to water the h h leaves its electron to the cl c l atom forming the clx− c l x − and the h h attaches to water to form the hydronium ion ( hx3ox+ h x 3 o x + ). The water dissociation constant remains the same whether the aqueous solution is neutral, acidic, or basic, i.e.: The above equation can then be used to calculate the molarity of the 70 wt % nitric acid: Through a process known as hydrolysis, the ions produced when an acid and base combine may react with the water molecules to produce a solution that is slightly acidic or basic. In reality, this reaction reaches an equilibrium. In this video we will look at the equation for hcl + h2o and write the products. As a general concept, if a strong acid is mixed with a weak base, the resulting solution will be slightly acidic.4.3 AcidBase Reactions Introduction to Chemistry
[DIAGRAM] Phase Diagram Hcl Water
Chemical Equation For The Acid Ionization Of Hydrochloric Hcl In Water
Complete The Chemical Equation For Acid Ionization Of Hydrochloric Hcl
Reaction of Hydrochloric Acid with Water, Chemistry Lecture Sabaq.pk
PPT Unit 7 Acids and Bases PowerPoint Presentation, free download
Hydrochloric acid molecule Stock Vector Images Alamy
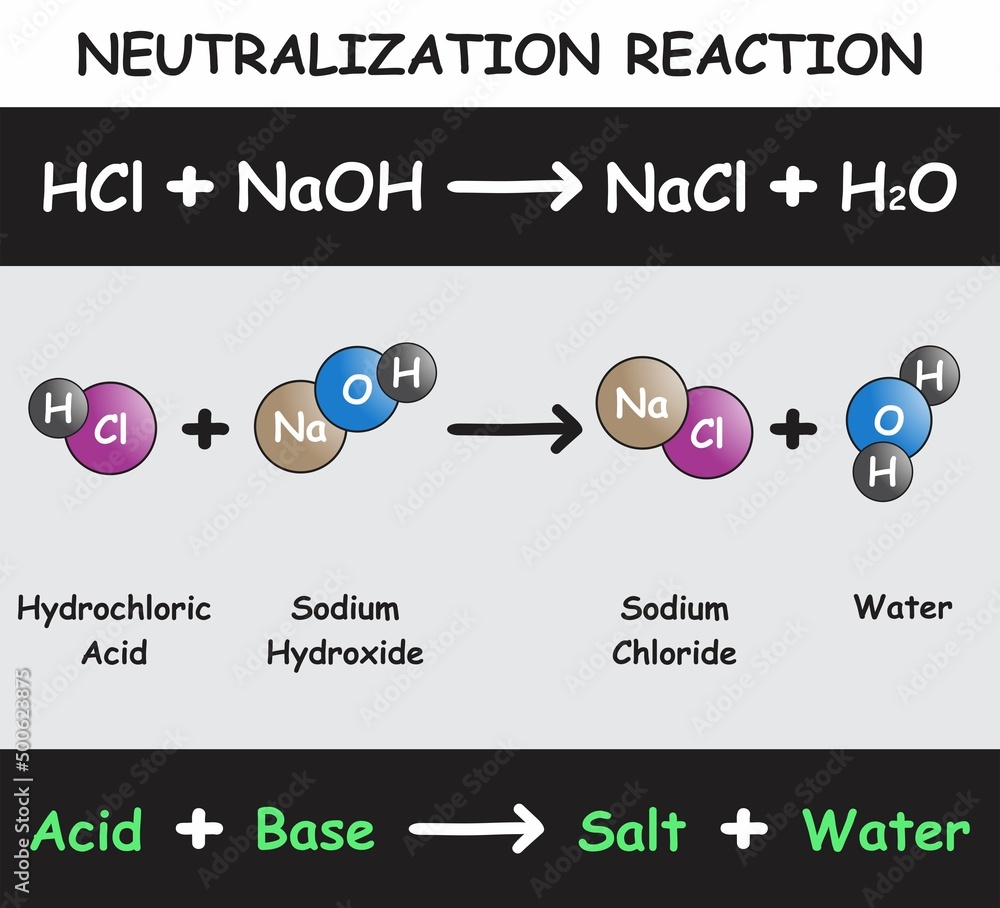
Neutralization Reaction Infographic Diagram with example of
What is the chemical equation for HCl dissolving into water and
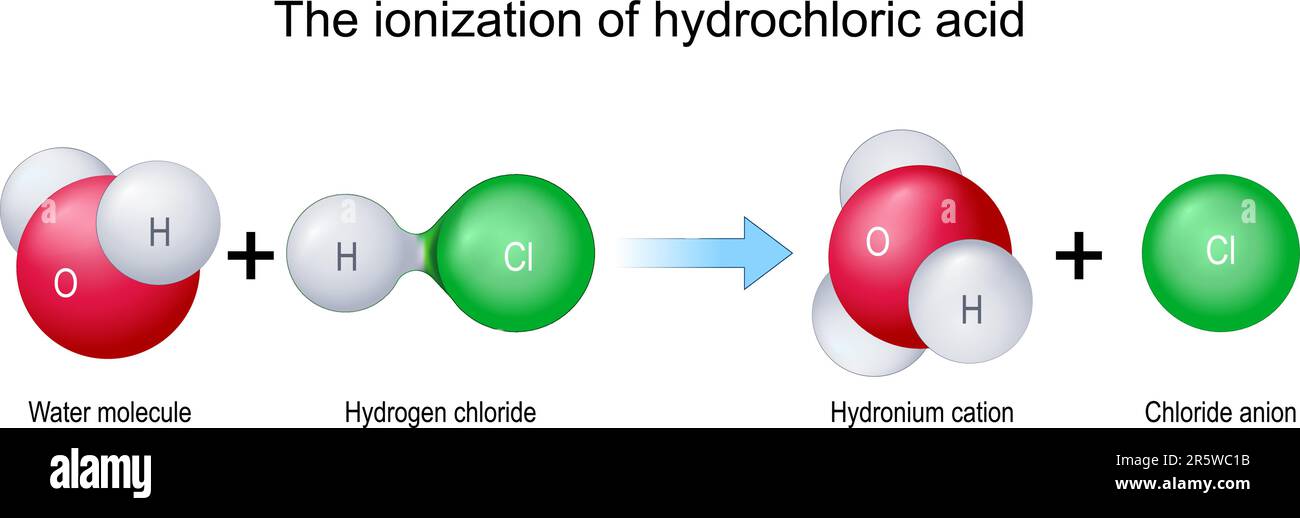
The ionization of hydrochloric acid. Molecules H2O and HCl combine to
Hydrochloric Acid, Hcl, Is A Strong Acid, So Right From The Start You Should Expect It To Ionize Completely In Aqueous Solution.
If You Paired Water With Something Which Is A Weaker Acid Than Water, Then Water Will Act As An Acid.
(I) Draw A Diagram To Show The Arrangement Used For The Absorption Of Hcl Gas In Water.
(Iii) Write The Chemical Equation For The Laboratory Preparation Of Hcl Gas When The Reactants Are:
Related Post:

![[DIAGRAM] Phase Diagram Hcl Water](http://c8.alamy.com/comp/H3N06T/diagram-of-the-laboratory-preparation-of-carbon-dioxide-from-hydrochloric-H3N06T.jpg)